Information Site About Reproductive System
About Me
Blog Archive
-
▼
2009
(58)
-
▼
December
(17)
- Syphilis During Pregnancy
- STDs and Pregnancy - Fact Sheet
- URINARY TRACT INFECTIONS in PREGNANCY
- GROUP B STREPTOCOCCUS INFECTION and PREGNANCY
- SWINE FLU and PREGNANCY
- GENITAL HERPES IN PREGNANCY
- LISTERIA and PREGNANCY
- REPIRATORY INFECTIONS DURING PREGNANCY
- HEPATITIS IN PREGNANCY
- CYTOMEGALOVIRUS
- CHLAMYDIA
- RUBELLA
- TOXOPLASMOSIS
- RECTOCELE
- CYSTOCELE
- PROLAPSE OF THE UTERUS
- PELVIC FLOOR
-
▼
December
(17)
Monday, November 30, 2009
The tissue that lines the interior of the uterus is called the endometrium.
Endometriosis is the growth of endometrial tissue (from the lining of the uterus) in places outside of the uterus, such as the ovaries, fallopian tubes and bowel.
Endometriosis can cause numerous symptoms, including painful periods and pain with sex, fertility problems, pelvic and ovulation pain, pain in the lower back and thighs, and bowel and bladder symptoms.
Treatment options include :
- SURGERY
- HORMONE TRATMENT and
- COMPLEMENTARY THERAPIES
Diagnosis can be difficult
Diagnosing endometriosis can be difficult, especially in the early stages of disease. The only way to diagnose the disease is to look inside the pelvic cavity, using a special instrument called a laparoscope. Diagnosis may be delayed if the woman assumes her degree of pain is normal and so doesn’t see her doctor. A diagnosis may also be delayed if the doctor is not familiar with the range of symptoms associated with endometriosis. If you have bad period pain, you should see your doctor.
Treatment options
The treatment options for endometriosis include:
- Observation with no medical intervention
- Hormone treatment
- Surgery
- Combined treatment
- Complementary therapies.
Observation with no medical intervention
In mild cases of endometriosis, it may be possible to simply monitor the condition with regular visits to your doctor or gynaecologist. Antiprostaglandin medications (non-steroidal anti-inflammatory drugs such as ibuprofen and mefenamic acid) can help to control any associated pain.
If symptoms progress, talk over the medical options with your health care provider before making a final decision. Remember that a mild condition can become moderate to severe. Removal of adhesions through surgery is the most effective treatment to lessen the chances of recurrence.
Hormone treatment
Each month, a woman’s uterine lining is prompted by the hormone oestrogen to thicken in preparation for possible pregnancy. During menstruation, the hormone progesterone causes the plump uterine lining to shed. The misplaced endometrial cells in other areas of the body also respond to oestrogen and progesterone. Hormone therapy can sometimes be an effective way to manage the symptoms of endometriosis.
Options for hormone therapy include:
- GESTRINONE – is a synthetic hormone that causes the endometriosis to become inactive and waste away. Side effects of gestrinone include weight gain, acne, depression, mood swings, hot flushes and loss of libido (sex drive).
- DYDROGESTERONE– a synthesised version of the hormone progesterone, which helps to dry up the stray endometrial cells. Ovulation may still occur. Side effects of dydrogesterone can include depression, tender breasts, weight gain, fatigue, nausea, headaches and dizziness.
- MEDROXYPROGESTERON ACETATE– another synthesised version of the hormone progesterone. Most women taking medroxyprogesterone acetate will stop ovulating and menstruating. Other side effects include weight gain, bloating, irregular vaginal bleeding, depression, sweating, headaches, acne, nausea, fatigue and tender breasts.
- GnRH AGONIST– gonadotrophin-releasing hormones help to govern the menstrual cycle. GnRH agonists are drugs that stop the ovaries from producing as much oestrogen by reducing the hormones produced by the pituitary gland (follicle stimulating hormone and luteinising hormone). Without oestrogen, the misplaced endometrial cells are unable to grow. Side effects of GnRH agonists include menopausal symptoms such as thinning of the bones, hot flushes, dry vagina, headaches, depression, loss of libido and night sweats. These symptoms can be relieved, while still maintaining the benefit of the treatment, by adding back oestrogen and progesterone.
- The oral CONTRACEPTIVE PILL– the pill is frequently used to achieve long-term suppression of endometriosis. It can be used to stop the disease progressing in women with mild disease or to stop the disease from recurring following surgical or hormonal treatment.
- DANAZOLE– this mild form of the male hormone testosterone reduces the amount of oestrogen produced by the ovaries to around the same level as occurs during menopause. Without oestrogen, the stray endometrial cells can’t grow and, therefore, shrink and disappear during the treatment. Danazol is now rarely used to treat endometriosis due to its serious side effects, which can include weight gain, bloating, fluid retention, acne, smaller breasts, increase in muscle mass, increased facial and body hair, muscle cramps, mood swings, voice deepening and clitoral enlargement. Danazol can also cause gastrointestinal upsets, depression and liver disease. These treatments can have side effects, so make sure you are well informed about them before you and your doctor decide on your treatment.
Surgery
The different types of surgery include:
- LAPAROSCOPIC SURGERY– a slender tube is inserted into the abdominal cavity via a small incision (also known as ‘keyhole’ surgery). Endometrial implants, cysts and adhesions are then removed by cutting out (excision), burning (cauterisation) or vapourising (ablation) them. Cutting out is the most effective of these methods. You should seek an expert in advanced laparoscopic surgery to conduct your procedure. Some doctors can perform surgery, including the removal of the ovaries or removal of endometriosis from the bowel, laparoscopically.
- LAPAROTOMY (open surgery) – a surgical incision into the abdominal cavity to cut out or burn tissue or remove cysts (with the advent of laparoscopic surgery, this procedure is now rarely necessary).
- BOWEL RESECTION – may be necessary if the bowel has developed endometriosis adhesions.
- HYSTERECTOMY – the uterus is removed, along with endometrial implants, cysts and adhesions. In some cases, the fallopian tubes and ovaries will also be removed. Unfortunately, hysterectomy does not always cure endometriosis.
Combined treatment
In some cases, a woman will benefit from undergoing hormone therapy as well as surgery. Hormone therapy may be offered before or after the surgery, depending on the circumstances.
Complementary therapies
Some women find complementary therapies to be helpful. Always tell your doctor about the kinds of complementary therapies you are using or considering. Options include:
- Acupuncture
- Chinese medicine
- Herbal therapy
- Homeopathy.
Pregnancy is not a cure
Some people believe that endometriosis can be cured by pregnancy. This isn’t the case. The symptoms may improve for some women, but worsen in others. For those women who experience an end to all symptoms during pregnancy, the relief may only be short lived. Unfortunately, for some women, the endometriosis will recur.
Labels: Dysmenorrhoea, Endometriosis, Infertility
The tissue that lines the inside of the uterus is called the endometrium. Endometriosis is the growth of endometrial tissue in places outside the uterus, such as the ovaries, uterus, bowel and lining of the pelvic cavity.
The causes of endometriosis remain unknown, but researchers have uncovered a number of possible causes and risk factors.
Endometriosis can cause numerous symptoms, including :
- Painful periods (DYSMENORRHOEA)
- Pain with sex (DYSPAREUNIA) ,
- Fertility problems,
- Pelvic and ovulation pain,
- Pain in the lower back and thighs, and
- Bowel and bladder symptoms.
Usually, endometriosis causes pain around the time of the period, but some women experience almost constant pain. If you have bad period pain, you should see your doctor.
Stray endometrial cells respond to hormones
The endometrium responds to the sex hormones oestrogen and progesterone. In women with endometriosis, the stray endometrial cells in the pelvic cavity also respond to these hormones.
During ovulation, oestrogen prompts the uterine lining – and the misplaced endometrial cells – to thicken. However, the misplaced endometrial cells cannot leave the body via menstruation; they simply bleed a little, causing inflammation and pain, and then heal. Over time, this may create scar tissue. Affected organs, such as the ovaries and bowel, may stick together. This can cause chronic pain and bowel symptoms. Sometimes, it can cause fertility problems if the scar tissue (adhesions) stops the released egg from getting to the fallopian tube.
Genetic susceptibility
Studies indicate that some women are genetically predisposed to developing endometriosis. According to researchers from the University of Queensland, endometriosis runs in families, which means the genetic susceptibility is inherited.
Australian researchers have found that women who have a sister with the disease are 2.3 times more likely to have the disease than women in the general community. The increased likelihood of developing the disease is not just confined to the daughters and sisters of women with the disease but also affects their cousins.
Possible causes
Some of the theories on what causes endometriosis include:
- Retrograde menstruation
- Immune system malfunction
- Genetic factors.
Retrograde menstruation
Retrograde menstruation is also known as ‘backward menstruation’. The lining of the uterus is shed during the period. In almost all women, some of the menstrual fluid flows backwards into the fallopian tubes instead of leaving the body through the vagina.
Since the fallopian tubes are open-ended (they are not joined to the ovaries, but open nearby), menstrual fluid can drip into the pelvic cavity. It is suspected that, in women who experience endometriosis, the endometrial tissue contained in the menstrual fluid sticks to whatever structures it lands on (such as the ovaries) and starts to grow.
Immune system malfunction
Retrograde menstruation occurs in almost all women, but only 3–10 per cent of menstruating women develop endometriosis. One theory suggests that the immune systems of some women allow endometriosis to develop by failing to control or stop the growth of endometrial tissue outside the uterus.
The genetic factor
It seems that genetic susceptibility plays a significant role in the development of endometriosis – but how? Some researchers suspect that some families carry faulty genes that allow abnormal cells to survive and grow in the pelvic cavity.
Risk factors
Apart from genetic susceptibility, some of the suspected risk factors include:
- MENSTRUAL CYCLE FACTORS– some evidence suggests that women with endometriosis are more likely to have started their periods at an early age. Other factors related to the menstrual cycle that may predispose a woman to endometriosis include heavy or painful periods, short menstrual cycles (less than 27 days) and long periods (more than one week).
- ALLERGIES – such as food, eczema and hay fever.
- OBESITY
- EXPOSURE TO TOXINS – persistent environmental pollutants, such as dioxins, are suspected of contributing to the development of endometriosis. Animal experiments have indicated such an effect, but at levels of exposure higher than those currently seen in humans.
Reducing your endometriosis risk
Factors that may help reduce your risk of endometriosis include:
- AEROBIC EXCERCISE of five hours per week – studies show a 50 per cent reduction in the risk of recurrence
- CHILDBEARING – reduces the risk of recurrence by about 50 per cent.
- The CONTRACEPTIVE PILL – this prevents ovulation, during which there is a surge of oestrogen production and spill into the pelvis.
Labels: Dysmenorrhoea, Endometriosis, Infertility
Endometriosis is a condition in which endometrium, the tissue that normally lines the womb (uterus), grows in locations outside the uterus.
Endometriosis may cause adhesions (fibrous scar tissue) on the uterus.
The uterus can become stuck to the ovaries, fallopian tubes and bowel.
The pain of endometriosis can be so bad that it stops you from going to work or school. Usually, it causes pain around the time of your period but for some women the pain is almost constant. If you need treatment, you may need emotional as well as physical support.
Symptoms
The symptoms of endometriosis include:
- Painful periods
- Pain with sex
- Pelvic pain
- Ovulation pain
- Pain in the lower back and thighs
- Bowel symptoms
- Bladder symptoms
- Reduced fertility
- Nausea and lethargy
- Pre-menstrual tension (PMT).
Many women think that painful periods are normal. If you have bad period pain, you should see your doctor.
Diagnosis
Tests that are used to help diagnose endometriosis include:
- LAPAROSCOPY– surgical procedure performed under general anaesthetic, where a medical instrument with a video camera attached is used to examine your pelvic organs.
- ULTRASOUND – a vaginal or abdominal instrument that uses sound waves to create a video image of your pelvic organs.
- COLONOSCOPY– a medical instrument with a video camera attached that is used to examine your bowel. This is done if it is thought that the endometriosis could also be affecting your bowel. You would be sedated for the procedure.
Endometriosis may not show up during an internal pelvic examination. Your doctor may need to refer you to a gynaecologist if you have endometriosis.
Treatment
Endometriosis can be treated medically (with drugs or medicine) or with surgery. Sometimes both medicine and surgery are used. Some women also benefit from natural therapies.
Drug therapy
Medications that are used to help treat endometriosis include:
- Anti-inflammatory medications
- Pain killers (Mersyndol is often used)
- Hormonal treatments, which suppress ovulation and menstruation and may have side effects. There are different categories of these such as GnRH agonists (Zoladex, Synarel), androgenic steroids (Danocrine, Azol, Dimetriose), progestogens (Provera, Duphaston, Primolut) and the oral contraceptive pill.
Surgery
Surgical methods used to treat endometriosis include:
- LAPAROSCOPIC SURGERY– performed to diagnose and treat endometriosis. Laser surgery may be used to remove the adhesions. This is done to reduce pain and improve the chances of you becoming pregnant.
- LAPAROTOMY – to cut out or burn tissue or remove cysts.
- BOWEL RESECTION – may be necessary if the bowel has developed endometriosis adhesions.
- HYSTERECTOMY(removal of the uterus) – may be an option if endometriosis is significantly impacting on your quality of life and other treatments have not worked.
If your ovaries are removed during a hysterectomy, you will need to discuss hormone replacement with your doctor.
Complementary therapies
There are many different forms of complementary therapies that can be used to treat endometriosis. Most therapies may be used in conjunction with Western medicine or instead of surgery and drug therapy.
Examples of different therapies include:
- Herbal medicine
- Homeopathy
- Traditional Chinese medicine
- Nutritional therapies
- Massage
- Yoga.
Labels: Dysmenorrhoea, Endometriosis, Infertility
Sunday, November 29, 2009
Adenomyosis
Known as "Endometriosis of the uterus," Adenomyosis is benign and does not cause cancer. Most commonly, the disease affects the back wall (posterior side) of the uterus. The endometrial cells penetrate deep into the uterine muscle (myometrium). When this occurs, the uterus is enlarged usually more than twice the normal size and very hard. The disease may be localized with well-defined borders or diffuse, meaning having no limits or borders. When this localized disease is found it is called adenomyoma. These adenomyomas can be located at different depths of the uterine muscle and can penetrate into the uterine cavity, becoming submucosal tumors
How common is Adenomyosis?
This disease can only be diagnosed with 100% certainty by doing a biopsy of the uterine muscle. Depending on the various reported studies published, it has been noted to occur in 8-62% of women who have had hysterectomies. 12% of women with Adenomyosis have also had Endometriosis in other sites such as the pelvic wall, ovaries, fallopian tubes etc. The highest incidence is seen in women in their mid to upper forties, and though this disease may cause infertility, it often appears in women who have already had children.
What are the symptoms of Adenomyosis?
As with Endometriosis, patients with Adenomyosis may not show any symptoms (asymptommatic). However, women most commonly experience excessive, heavy or prolonged menstrual bleeding and painful periods (dysmenorrhea). The amount of bleeding and cramps is usually associated with the degree of disease involvement and depth of penetration into the uterine walls. Extensive involvement of the uterine muscle can also interfere with the normal contractility of the muscle which then leads to excessive bleeding.
How is Adenomyosis diagnosed?
An exact diagnosis is often difficult to establish pre-operatively because abnormal patterns of bleeding (dysfunctional bleeding) and fibroid tumors can result in similar symptom patterns. Sometimes during a D&C procedure to remove intra-uterine polyps or small fibroid tumors, tissue is removed enabling a pathologist to make the diagnosis.
Pelvic Exam Findings
Pelvic exam findings can reveal a normal, or only slightly enlarged uterus to a very firm tender uterus enlarged to twice the normal size.
MRI- Magnetic Resonance Imaging
At times, this can distinguish adenomyomas from fibroid tumors, but again, experienced physicians and radiologists possessing extensive training are required.
MRI provides better diagnostic capability due to the increased spatial and contrast resolution, and to not being limited by the presence of bowel gas or calcified uterine fibroids (as is ultrasound). In particular, MR is better able to differentiate adenomyosis from multiple small uterine fibroids. The uterus will have a thickened junctional zone with diminished signal on both T1 and T2 weighted sequences due to susceptibility effects of iron deposition due to chronic microhemorrhage. A thickness of the junctional zone greater than 10 to 12 mm (depending on who you read) is diagnostic of adenomyosis (<8 mm is normal). Interspersed within the thickened, hypointense signal of the junctional zone, one will often see foci of hyperintensity (brightness) on the T2 weighted scans representing small cystically dilatated glands or more acute sites of microhemorrhage.
MR can be used to classify adenomyosis based on the depth of penetration of the ectopic endometrium into the myometrium.
Transvaginal Ultrasound
Extensive recent work has been completed with this test, but the amount of false positive results is still high.
Tissue Diagnosis
Tissue diagnosis in some form remains the only definitive method for diagnosing Adenomyosis. If the diagnosis is suspected pre-operatively, then a laparoscopy and a long needle biopsy can be performed, whereby a needle is inserted into the back of the uterus to collect a tissue sample for pathological testing. It may also be diagnosed when fibroid tumors are removed .
Can Adenomyosis be treated without surgery?
Some studies have shown that there is a relationship between Adenomyosis and hormone imbalance, most commonly an excess of estrogen. Progesterone therapy, either in the natural or synthetic form has been known to help, but shows very little long term benefits. A medication called Danazol may be helpful in treating the pain and decreasing the size of the uterus but long term positive results are poor. Although gonadotropin-releasing hormone agonists such as Lupron have been found to reduce uterine symptoms of adenomyosis during treatment, the symptoms return quickly after the medicine wears off.
How can Female Alternative Surgery help Adenomyosis?
Most commonly, hysterectomy has been the mainstay of treatment. Traditional medicine states that since most women with Adenomyosis are beyond child-bearing age, the uterus is no longer relevant. At the Institute, we want to give women every opportunity to retain their female organs even if fertility is not a concern. Our surgical approach is first to make a diagnosis. For women who still wish to conceive, we try to remove the Adenomyosis using laser technology (CO2 Yag and Argon) which preserves the endometrial cavity but treats the remaining deep uterine muscle disease. In the case of women who are not concerned with fertility but want to preserve their organs, our approach is to remove as much of the affected tissue and, if necessary, decrease the size of the endometrial cavity. We treat the remaining uterine muscle with a deep tissue laser technique. Post surgical results have shown that pain almost always disappears and menstrual flow and volume decrease.
Labels: Dysmenorrhoea, Endometriosis, Infertility
Saturday, October 31, 2009
 In vitro fertilisation (IVF) is the process used to conceive a child outside the body. A woman’s eggs and a man’s sperm are placed together in a plastic dish for fertilisation. Once fertilised, the resulting embryos are placed back in the woman’s uterus in the hope that a successful pregnancy will follow.
In vitro fertilisation (IVF) is the process used to conceive a child outside the body. A woman’s eggs and a man’s sperm are placed together in a plastic dish for fertilisation. Once fertilised, the resulting embryos are placed back in the woman’s uterus in the hope that a successful pregnancy will follow.
The IVF procedure
IVF is not one simple procedure, but a series of steps over several weeks. The steps involved in this procedure are outlined below.
Stimulating the ovaries
Hormones are usually given to stimulate the ovaries to produce more than the usual one egg per cycle. This is to enable the collection of several eggs.The development of the eggs is monitored by one or two blood tests and ultrasounds. The ultrasound and blood tests ensure that eggs are collected at precisely the right time.
Collecting the eggs
 The ultrasound is inserted in the vagina and a very fine needle is threaded through a guide, which is attached to a probe. Only a light anaesthetic is required for this procedure. The ultrasound monitor shows where the follicles are. The needle pierces the follicle and extracts the follicular fluid, which contains the egg.
The ultrasound is inserted in the vagina and a very fine needle is threaded through a guide, which is attached to a probe. Only a light anaesthetic is required for this procedure. The ultrasound monitor shows where the follicles are. The needle pierces the follicle and extracts the follicular fluid, which contains the egg.
Fertilisation and embryo transfer
A couple of hours after egg collection, the man provides a sample of semen. In a standard IVF treatment, the eggs are mixed with the sperm in a culture dish. For intracytoplasmic sperm injection (ICSI) treatment, one sperm is injected directly into the cytoplasm of each egg.
If an egg is fertilised by a sperm, a zygote or pre-embryo will begin to develop. The pre-embryo remains in the incubator for one or two days, until it has divided into two or four cells. Following fertilisation, two to three embryos will be transferred to the uterus using a soft, fine catheter. This procedure (known as embryo transfer) is quite painless, similar to a smear test, and requires no anaesthetic.
For the gamete intrafallopian transfer (GIFT) program, eggs and sperm are placed directly into the fallopian tubes, allowing fertilisation to take place in the natural way. The procedure is performed using a laparoscope, and a general anaesthetic is required. This procedure is rarely used now.
Pregnacy test results
Two weeks after the transfer, a blood test is taken to determine if the woman is pregnant.
Possible risks and side effects
There is no clear evidence that infertility medicines, if properly used, increase the risk of birth defects or cancer. The increase in the hormone oestrogen can cause breast tenderness, slight nausea, dizziness and slight abdominal swelling. Occasionally, too many follicles develop and a condition called ovarian hyperstimulation syndrome (OHSS) may occur. This is an unpleasant experience, which may include marked abdominal swelling, nausea, vomiting and diarrhoea, lower abdominal pain and shortness of breath. There is also a theoretical risk (very rare) of damaging organs, or causing infection or bleeding, with the collection needle.
Things to remember
- IVF is a process where fertilisation of an egg occurs outside of the body.
- IVF is not one procedure but rather a series of steps taken over several weeks.
- While infertility drugs have some side effects, there is no evidence that they cause cancer or birth defects.
Labels: Infertility
Friday, October 30, 2009
Semen analysis
A semen analysis evaluates certain characteristics of a male's semen and the sperm contained in the semen. It may be done while investigating a couple's infertility or after a vasectomy to verify that the procedure was successful.
Relation to fertility
The characteristics measured by semen analysis are only some of the factors in semen quality. One source states that 30% of men with a normal semen analysis actually have abnormal sperm function.[1] Conversely, men with poor semen analysis results may go on to father children.[2][3]
Collection methods
The most common way to collect a semen sample is through masturbation, directing the sample into a clean cup.[2]
A sample may also be collected during intercourse in a special type of condom known as a collection condom. Collection condoms are made from silicone or polyurethane, as latex is somewhat harmful to sperm.[4] Many men prefer collection condoms to masturbation, and some religions prohibit masturbation entirely. Adherents of religions that prohibit contraception, such as Catholicism, may use collection condoms with holes pricked in them.[5]
A third option for collecting a sample is through coitus interruptus (withdrawal). With this technique, the man removes his penis from his partner near the end of intercourse and ejaculates into a cup.
Finally, if a blockage in the vas deferens is suspected to impede fertility, semen can be taken directly from the epididymis. Such a collection is called per cutaneous epididymal sperm aspiration (PESA). Alternatively, the testicular tissue itself, instead of the sperm produced can be investigated. Then, the collecting method is called TESE.[6]
Tested characteristics
Examples of parameters measured in a semen analysis are: sperm count, motility, morphology, volume, fructose level and pH.
1. Sperm count
Approximate pregnancy rate varies with amount of sperm used in an artificial insemination cycle. Values are for intrauterine insemination, with sperm number in total sperm count, which may be approximately twice the total motile sperm count.
Sperm count, or sperm concentration to avoid mixup, measures the concentration of sperm in a man's ejaculate, distinguished from total sperm count, which is the sperm count multiplied with volume.[7] Anything over 20 million sperm per milliliter is considered normal.[1][2] Anything less is considered oligospermia. A vasectomy is considered successful if the sample is azoospermic. Some define success with rare non-motile sperm are observed (fewer than 100,000 per millilitre).[8] Others advocate obtaining a second semen analysis to verify the counts are not increasing (as can happen with re-canalization) and others still may perform a repeat vasectomy for this situation.
The average sperm count today is around 60 million per milliliter in the Western world, having decreased by 1-2% per year from a substantially higher number decades ago.[9]
2. Motility
The motility of the sperm is evaluated. WebMD defines normal motility as 60% of observed sperm, or at least 8 million per millilitre, showing good forward movement.[2] The World Health Organization has a similar value of 50% and this must be measured within 60 minutes of collection. A man can have a total number of sperm far over the limit of 20 million sperm cells per milliliter, but still have bad quality because too few of them are motile. However, if the sperm count is very high, then a motility of less than 60% might not matter, because the fraction might still be more than 8 million per millilitre. The other way around, a man can have a sperm count far less than 20 million sperm cells per millilitre and still have good motility, if more than 60% of those observed sperm cells show good forward movement.
A more specified measure is motility grade, where the motility of sperm are divided into four different grades:[10]
- Grade 4: Sperm with progressive motility. These are the strongest and swim fast in a straight line. Sometimes it is also denoted motility a.
- Grade 3: (non-linear motility): These also move forward but tend to travel in a curved or crooked motion. Sometimes also denoted motility b.
- Grade 2: These have non-progressive motility because they do not move forward despite the fact that they move their tails.
- Grade 1: These are immotile and fail to move at all.
3. Morphology
The morphology of the sperm is also evaluated. With WHO criteria as described in the old manual of 1989, a sample is normal if 30% or more of the observed sperm have normal morphology.[1] If morphology is evaluated using the Tygerberg strict criteria developed by Dr. Roelof Menkveld, Tygerberg Hospital, South Africa, and disseminated by Dr. Thinus Kruger from the same hospital,[11] a sample is normal if 14% or more of the observed sperm have normal morphology.[1]. The Tygerberg strict criteria for morpology assessment are recommended in the most recent WHO manual on semen analysis (WHO 1999). According to the above references, morphology was developed as a predictor of success in fertilizing oocytes during invitro fertilization.
4. Volume
The volume of the sample is measured. WebMD advises that volumes between 1.0 mL and 6.5 mL are normal;[2] WHO criteria specify that any volume greater than 2.0 mL is normal. Low volume may indicate partial or complete blockage of the seminal vesicles, or that the man was born without seminal vesicles.[1] In clinical practice, a volume of less than 2 mL in the setting of infertility and absent sperm should prompt an evaluation for obstructive azoospermia. A caveat to this is be sure it has been at least 48 hours since the last ejaculation to time of sample collection.
5. Fructose level
The level of fructose in the semen is measured. WebMD lists normal as at least 3 mg/mL.[2] WHO specifies a normal level of 13 μmol per sample. Absence of fructose may indicate a problem with the seminal vesicles.[1]
6. pH
The pH of the sample is measured. WebMD lists a normal range of 7.1-8.0;[2] WHO criteria specify normal as 7.2-7.8.[1] Acidic ejaculate (lower pH value) may indicate one or both of the seminal vesicles are blocked. A basic ejaculate (higher pH value) may indicate an infection.[1] A pH value outside of the normal range is harmful to sperm.[2]
7. Liquefaction
The liquefaction is the process when the gel formed by proteins from the seminal vesicles is broken up and the semen becomes more liquid. It normally takes less than 20 minutes for the sample to change from a thick gel into a liquid. An abnormally long liquefaction (more than 30 minutes at 37 24°C) time may indicate an infection.
8. MOT
MOT is a measure of how many million sperm cells per ml are highly motile[12], that is, approximately of grade 4, or sometimes also taking grade 3 into account. Thus, it is a combination of sperm count and motility.
With a straw volume of 0.5 milliliter per straw, the general guideline is that, for intracervical insemination (ICI), straws making a total of MOT40 is recommended. This is equal to 8 straws with MOT5, or 2 straws of MOT20. For intrauterine insemination (IUI), straws making a total of MOT10 is regarded sufficient.[13] In WHO terms, it is thus recommended to use approximately 20 million grade 3+4 sperm in ICI, and 5 million ones in IUI.
Total motile spermatozoa
Total motile spermatozoa (TMS)[14] or total motile sperm count (TMSC)[15] is a combination of sperm count, motility and volume, measuring how many million sperm cells in an entire ejaculate are motile.
Use of approximately 20 million grade 3+4 sperm in ICI, and 5 million ones in IUI may be an approximate recommendation.
9. Others
The sample is tested for white blood cells. A high level of white blood cells (over 1 million per milliliter) may indicate an infection.[1]
Abnormalities
- Aspermia: absence of semen
- Azoospermia: absence of sperm
- Oligospermia: low number of sperm
- Asthenozoospermia: poor sperm motility
- Teratozoospermia: sperm carry more morphological defects than usual
Factors that influence results
Compared to samples obtained from masturbation, semen samples from collection condoms have higher total sperm counts, sperm motility, and percentage of sperm with normal morphology. For this reason, they are believed to give more accurate results when used for semen analysis.[4]
How long the man has abstained prior to providing the sample for analysis affects the results. Longer periods of abstinence correlate with poorer results - one study found that men with repeated normal results produced abnormal samples if they abstained for more than 10 days. It is recommended not to abstain for more than one or two days before providing the semen sample for analysis.[16]
If the results from a man's first sample are subfertile, they must be verified with at least two more analyses. At least 2 to 4 weeks must be allowed between each analysis.[17] Results for a single man may have a large amount of natural variation over time, meaning a single sample may not be representative of a man's average semen characteristics.[18] In addition, sperm physiologist Joanna Ellington believes that the stress of producing an ejaculate sample for examination, often in an unfamiliar setting and without any lubrication (most lubricants are somewhat harmful to sperm), may explain why men's first samples often show poor results while later samples show normal results.[3]
A man may prefer to produce his sample at home rather than at the clinic. The site of semen collection does not affect the results of a semen analysis.[19]
Measurement methods
Volume can be determined by measuring the weight of the sample container, knowing the mass of the empty container. Sperm count and morphology can be calculated by microscopy. Sperm count can also be estimated by kits that measure the amount of a sperm-associated protein, and are suitable for home use. [20]
Computer Assisted Semen Analysis (CASA) is a catch-all phrase for automatic or semi-automatic semen analysis techniques. Most systems are based on image analysis, but alternative methods exist such as tracking cell movement on a digitizing tablet.[21][22] Computer-assisted techniques are most-often used for the assessment of sperm concentration and mobility characteristics, such as velocity and linear velocity. Although many systems can give very accurate information about motility patterns of motile sperm, immotile sperm cannot accurately be distinguished from other cells, particles or debris without addition of staining. The first CASA systems in general cannot give reliable results for sperm concentration and proportions of sperm with different grades of motility, at least not in semen samples, where there are many other particles, cells and debris. Even when it comes to motility, most systems cannot give reliable results when the concentration of motile sperm leads to significant "crossed paths" - due to difficulties to decide if there were two sperm crossing each others way, or if there were for instance four sperm moving close to each other. Nowadays, there are CASA systems, based on image analysis and using new techniques, with near perfect results, and doing full analysis in a few seconds.
CASA Systems
SQA-V - The SQA-V, also known as the 'Sperm Quality Analyzer or Spermalite, is a high performance sperm analysis instrument used to test male fertility. It combines electro-optics, computer algorithms and video microscopy to provide a precise and accurate 75 second automated semen analysis. This device is manufactured by Medical Electronic Systems [23], who specializes in rapid, automated semen analysis. The main reason the SQA-V is a growing instrument among the semen analysis community is due to the speed, accuracy, and precision to run a semen sample. In addition, the SQA-V semen analysis eliminates inter-operator variables from the manual method, and still provides 16 clinical parameters including: Sperm Concentration, Rapid Progressive motility, Slow Progressive motility, Non-progressive motility, Immotility, %Normal Morphology, and more.
A study was conducted by the world renowned Cleveland Clinic comparing the SQA-V to the Manual method and the CASA device, presenting results favoring the SQA-V. [24]
ISAS - Integrated Semen Analysis System is a CASA system based on image analysis from the company Projects i Serveis R+D S.L., also known as PROISER. PROISER was founded on 2004 by a team of CASA developers (SCA 96, SCA 98 and SCA 2002) with more than 15 years in experience of seminal analysis by computer image analysis. ISAS can be considered as the most complete and easiest-to-use system in market which, furthermore, works in different hardware and operating-system conditions, in order to adapt as much as possible to the needs of our users. Current computers and AAVT technology allows a very good matching of spermatozoon, thanks to the tail and morphology analysis made at the same time as motility analysis. Only motility and concentration analysis give to the customers more tan 17 sperm parameters, but also ISAS analyzes automatically stained morphometry, giving 15 parameters and DNA fragmentation analysis. ISAS has been developed to be used in several species, from classical species like human, boar or bull to new research species like cod or some small rodents. There are a lot of publications and works in progress with ISAS like [25]. A list of last publications with this CASA system can be found on publications with ISAS
Sperm Class Analyzer - The Sperm Class Analyzer, also known as 'SCA' from the company Microptic S.L., provides fast, accurate and objectively repeatable results. This would be impossible to attain using traditional (subjective) methods. This CASA system has the following modules: SCA Motility & Concentration: The system provides immediately and objectively detailed results of motility and concentration in a complete report. SCA Morphology: Following a manual or automatic image capture, a precise morphological and morphometric analysis of each spermatozoid is provided in real time. SCA DNA Fragmentation: Analysis of halo formation of the samples prepared for study of the DNA fragmentation provides detailed numerical data for each of the cells. SCA Vitality: Automatically analyze the vitality of a sperm sample. All this modules can be used with a motorized stage or link the database with any hospital system.
IVOS Sperm Analyzer - The Integrated Visual Optical System (IVOS) was developed by Hamilton Thorne, the leading manufacturer of CASA systems since 1986. The IVOS is found in hospitals, universities, IVF clinics, pharmaceutical companies, contract labs, reproductive toxicology labs, veterinary clinics and animal breeding facilities around the world. The standard IVOS software may be used to analyze sperm of multiple species, with a specific program geared toward analysis of rat sperm. The IVOS is unique in that it is the only CASA system that integrates the optical system within the unit, so that an external microscope is not needed. The light source of the IVOS produces stroboscopic illumination to provide blur free sperm images. This stroboscopic light source is especially beneficial when utilizing the IDENT fluorescent option on the IVOS as it allows the analysis of motile sperm under fluorescent illumination without adverse affect on sperm motility or velocity (this is not possible with continuous light fluorescent microscopes). Analysis using the IVOS IDENT provides highest accuracy of sperm counts in samples with a high degree of detritus, such as occurs in egg yolk extended samples or naturally in some species, such as rabbits. Samples are placed on a computer-controlled, heated stage which maintains samples at the proper analysis temperature and automatically moves to selected fields for analysis. A field of sperm are analyzed in just 0.5 second with a level of accuracy and repeatability unobtainable by visual assessment. Results calculated include count, concentration, motility, progressive motility, curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), linearity (LIN), straightness (STR), amplitude of lateral head displacement (ALH) and beat cross frequency (BCF). Custom software packages are available, such as Animal Breeder, Equine Breeder and Animal Motility, which provide additional features specific to the target industries. In addition, the TOX IVOS is a complete system configured specifically for the inticacies of rat sperm analysis. Two automated morphology options, Dimesnisons Strict Morphology (developed and validated by Dr. Thinus Kruger) and Metrix user defined morphology are available. Another fluorescent option is VIADENT, which permits the analysis of motility and viability on the same live sample.
"Our results suggest that the VIADENT option of the IVOS system is capable of a rapid, accurate and objective evaluation of both viability and motility parameters using large numbers of spermatozoa. Application of this technique in the industry may prove useful in the clinical assessment of fertilizing potential of equine spermatozoa." [26]
CEROS Sperm Analyzer - Also from Hamilton Thorne and built upon the same analysis algorithms and software interface as the IVOS, the CEROS offers the same level of accuracy and reliability for sperm analysis. The CEROS uses an external negative phase contrast microscope for image visulaization and analysis. The system comes complete with motility analysis software, microscope, camera, computer and monitor. The CEROS is also compatible with both the Dimensions and Metrix morphology options and can be used for to analyze sperm from all species except rat. Both systems offer 4 levels of quality control as well as high-level security and audit trail for data inetgrity. A searacble list of publications featuring the Hamilton Thorne IVOS and CEROS CASA systems is found on Connotea.
References
- "Understanding Semen Analysis". Stonybrook, State University of New York. 1999. http://www.uhmc.sunysb.edu/urology/male_infertility/SEMEN_ANALYSIS.html. Retrieved 2007-08-05.
- Essig, Maria G.; Edited by Susan Van Houten and Tracy Landauer, Reviewed by Martin Gabica and Avery L. Seifert (2007-02-20). "Semen Analysis". Healthwise. WebMD. http://www.webmd.com/infertility-and-reproduction/guide/semen-analysis?page=1. Retrieved 2007-08-05.
- Ellington, Joanna (2004). "Understanding a Sperm Analysis". INGfertility. http://www.preseed.com/FAQs/FAQ4.php. Retrieved 2008-06-28.
- Dr. Joanna Ellington (January 2005). Use of a Specialized Condom to Collect Sperm Samples for Fertility Procedures. INGfertility. http://www.preseed.com/FAQs/FAQ4.php#Using_a_Sperm_Collection_Condom. Retrieved 2008-06-28.
- Kippley, John; Sheila Kippley (1996). The Art of Natural Family Planning (4th addition ed.). Cincinnati, OH: The Couple to Couple League. pp. pp.306–307. ISBN 0-926412-13-2.
- Fertility Center, Stockholm (translated from Swedish)
- sharedjourney.com - Male Infertility Testing
- Rajmil O, Fernández M, Rojas-Cruz C, Sevilla C, Musquera M, Ruiz-Castañe E (2007). "Azoospermia should not be given as the result of vasectomy" (in Spanish; Castilian). Arch. Esp. Urol. 60 (1): 55–8. PMID 17408173.
Dhar NB, Bhatt A, Jones JS (2006). "Determining the success of vasectomy". BJU Int. 97 (4): 773–6. doi:10.1111/j.1464-410X.2006.06107.x. PMID 16536771. - The sperm count has been decreasing steadily for many years in Western industrialized countries: Is there an endocrine basis for this decrease? The Internet Journal of Urology TM. ISSN: 1528-8390
- Shared Journey: Semen Analysis
- "Semen analysis morphology". IVF 1. 2005-10-27. http://www.ivf1.com/Semen-analysis-morphology/. Retrieved 2007-08-05.
- Cryos FAQs - What does MOT mean?
- Cryos FAQs - What is the recommended quantity and quality by ordering of donor semen?
- Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H (November 2008). "Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature". Fertil. Steril.. doi:10.1016/j.fertnstert.2008.09.058. PMID 18996517.
- Pasqualotto EB, Daitch JA, Hendin BN, et al. (October 1999). "Relationship of total motile sperm count and percentage motile sperm to successful pregnancy rates following intrauterine insemination". J. Assist. Reprod. Genet. 16 (9): 476–82. doi:10.1023/A:1020598916080. PMID 10530401. http://www.kluweronline.com/art.pdf?issn=1058-0468&volume=16&page=476.
- Ellington, Joanna (2005). "How Long to Abstain for a Sperm Test/Analysis". INGfertility. http://preseed.com/FAQs/FAQ1.php#How_Long_to_Abstain_for_a_Sperm_Test/Analysis__. Retrieved 2008-06-28. , which cites:
- Levitas E, Lunenfeld E, Weiss N, et al. (June 2005). "Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples". Fertil. Steril. 83 (6): 1680–6. doi:10.1016/j.fertnstert.2004.12.045. PMID 15950636.
- Jurema MW, Vieira AD, Bankowski B, et al. (September 2005). "Effect of ejaculatory abstinence period on the pregnancy rate after intrauterine insemination". Fertil. Steril. 84 (3): 678–81. doi:10.1016/j.fertnstert.2005.03.044. PMID 16169402.
- Weschler, Toni (2002). Taking Charge of Your Fertility (Revised ed.). New York: HarperCollins. pp. p.189. ISBN 0-06-093764-5.
- "Adequate Analysis Frequency". Kokopelli Technologies. 2007. http://www.fertilityformen.com/info_why.php#frequency. Retrieved 2007-08-11.
- Shetty Licht R, Handel L, Sigman M (2007). "Site of semen collection and its effect on semen analysis parameters". Fertil Steril. 89: 395. doi:10.1016/j.fertnstert.2007.02.033. PMID 17482174.
- dailyprogress.com > Charlottesville company sends out its home male sterility tests By Brian McNeill. Published: May 14, 2009
- Mortimer ST (01 Jul 2000). "CASA--practical aspects". J. Androl. 21 (4): 515–24. PMID 10901437. http://www.andrologyjournal.org/cgi/reprint/21/4/515. Retrieved 2007-08-05.
- Hinting A, Schoonjans F, Comhaire F (1988). "Validation of a single-step procedure for the objective assessment of sperm motility characteristics". Int. J. Androl. 11 (4): 277–87. doi:10.1111/j.1365-2605.1988.tb01001.x. PMID 3170018.
- http://mes-ltd.com/overview.asp
- Agarwal A, Sharma R (2007). "Automation is the key to standardized semen analysis using the automated SQA-V sperm quality analyzer". Fertility and Sterility 87 (No. 1): 156. doi:10.1016/j.fertnstert.2006.05.083. http://www.clevelandclinic.org/ReproductiveResearchCenter/docs/agradoc230.pdf.
- L. Ramió, M.M. Rivera, A. Ramírez, I.I. Concha, A. Peña, T. Rigau and J.E. Rodríguez-Gil (2008). "Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to in vitro capacitation and further in vitro acrosome reaction". Theriogenology 69 (No. 4): 501. doi:10.1016/j.theriogenology.2007.10.021. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCM-4RCNPPD-1&_user=10&_coverDate=03%2F01%2F2008&_rdoc=15&_fmt=summary&_orig=browse&_srch=doc-info(%23toc%235174%232008%23999309995%23679613%23FLA%23display%23Volume)&_cdi=5174&_sort=d&_docanchor=&_ct=17&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=9926ba71bee609b30a7c56f73857f511.
- Wessel MT, Althouse GC. Validation of an objective approach for simultaneous assessment of viability and motility of fresh and cooled equine spermatozoa. Proceedings of the International Symposium on Equine Reproduction, Animal Reproduction Science 94 (2006) 21-22
Labels: Infertility
Approximately one in five couples have trouble conceiving a child. If a couple is unable to conceive after one year of unprotected intercourse, they are said to be subfertile. Around 40 per cent of fertility problems originate in the man, with causes including sperm abnormalities and blockages within structures of the reproductive system, such as the vas deferens. Many men have sufficient sperm to fertilise their partner's eggs in a test tube, even if they are unable to do so during sexual intercourse. In most cases, the couple can be helped with assisted reproductive technologies. For around one in 10 couples investigated for infertility, no cause is found. This is known as 'idiopathic infertility'.
Diagnosis methods
Investigating suspected infertility requires a range of tests for both the man and his partner. Some of the tests the man may undergo include:
- Physical examination - including medical history.
- Ultrasound scans - to check the health of reproductive organs.
- Blood tests - to check sex hormone levels.
- Semen analysis - a sperm sample is checked for abnormalities and antibodies.
- Testicular biopsy - the fine network of tubes within the testicles is checked for the presence of sperm.
Some of the reproductive technologies available to infertile men include:
- Surgery
- Hormone therapy
- Artificial insemination
- In vitro fertilisation (IVF)
- Intra cytoplasmic sperm injection (ICSI).
Surgery
Fertility may be impaired by varicocele, or the bloating of veins inside the testes. This condition can be surgically treated. The tubes within the male reproductive system that transport sperm may be blocked, perhaps by injury or vasectomy. In some cases, the blockage can be surgically removed or the tubes repaired. If this doesn't work, the man may undergo another surgical procedure called percutaneous epididymal sperm aspiration (PESA). Under local anaesthetic, a slender needle is inserted into the epididymis, which is the tube at the back of the testicle that collects and stores sperm. Sperm is removed, and either used immediately for IVF or frozen.
Hormone therapy
The pituitary gland in the brain releases the hormone gonadotropin, which prompts the testicles to produce sperm. In a small number of cases, male infertility is caused by insufficient levels of gonadotropin. Taking a synthesised version of this hormone can boost sperm production.
Artificial insemination
The man's semen is collected, washed and concentrated, then introduced (via instruments) into his partner's vagina, cervix, uterus or fallopian tubes, depending on the circumstances. This option is often chosen if the husband has functional problems (such as impotence), or if his sperm can't make it through the cervix to the uterus. Some of the factors that can stall sperm at the entrance to the uterus include:
- The man's seminal fluid contains antibodies that destroy his sperm
- The cervical mucus contains antibodies that destroy his sperm
- The cervical mucus is so acidic that sperm are unable to survive.
In vitro fertilisation (IVF)
In vitro fertilisation (IVF) is conception within a test tube (or similar). The woman undergoes ovulation induction (hormonal stimulation of her ovaries) and a number of eggs are removed. This is done through the vagina under ultrasound control. The collected eggs are then mixed with sperm previously collected from the woman's partner, and placed in a special incubator. The fertilised eggs are then implanted into the woman's uterus via a thin tube inserted through the cervix.
Intra cytoplasmic sperm injection (ICSI) Sometimes, semen contains too few sperm to make fertilisation possible through IVF. In this case, intra cytoplasmic sperm injection (ICSI) can be used. The eggs are removed from the woman's ovaries, then individually injected with a single sperm each. When the eggs are fertilised, the embryos are transferred into the uterus.
Sometimes, semen contains too few sperm to make fertilisation possible through IVF. In this case, intra cytoplasmic sperm injection (ICSI) can be used. The eggs are removed from the woman's ovaries, then individually injected with a single sperm each. When the eggs are fertilised, the embryos are transferred into the uterus.


Figure 1:
Immobilizing the sperm's tail before picking it up.
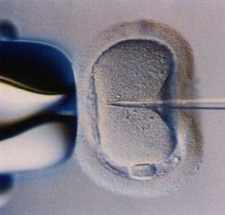
Figure 2:
Injection of sperm into the egg.

Figure 3:
Fertilized egg demonstrating the two nuclei – one from the father, one from the mother.
Pregnancy isn't always possible
Despite the sophistication of assisted reproductive technologies, pregnancy doesn't always happen. It depends on a range of factors, including the type of technology used and the reasons for the man's infertility. It should be remembered that assisted reproductive technologies can't improve the quality of sperm. Azoospermia, for example, means that the man's semen doesn't contain any sperm at all. In such cases, donor insemination may be considered. This involves artificially inseminating the partner with sperm from an anonymous donor.
Things to remember
- Around 40 per cent of fertility problems originate in the man, with causes including sperm abnormalities and blockages within structures of the reproductive system, such as the vas deferens.
- Some of the reproductive technologies available to infertile men include surgery to clear blockages, hormone therapy, artificial insemination, in vitro fertilisation (IVF) and intra cytoplasmic sperm injection (ICSI).
Labels: Infertility
New life begins when an egg from a woman is fertilised by sperm from a man. Around 20 million sperm per millilitre (ml) need to be present in the ejaculate, with enough mobility and strength to swim the journey to the fallopian tube, where conception normally takes place. The odds of a young fertile couple conceiving by having sexual intercourse around the time of ovulation are approximately one in five every month. A couple isn’t suspected of fertility problems until they have tried, and failed, to conceive for one year. Approximately 20 per cent of couples experience difficulties. In most cases, the couple can be helped with assisted reproductive technologies. Around 40 per cent of fertility problems originate in the man. Male fertility problems include poor quality sperm or blockages in the tubes of the reproductive system.
Obstructions
Sperm are made in the testicles. During ejaculation, sperm are pushed (by muscular contractions) through a series of small tubes called the epididymis, and mixed with seminal fluid from structures called seminal vesicles. The prostate gland also adds fluid. The semen is forced along a larger tube (vas deferens), into the urethra and out of the penis. In around one in three cases of male infertility, blockages or absences of tubes (including the vas deferens) are the cause of infertility. Causes may include vasectomy and injury.
Problems with sperm

- Absent sperm (azoospermia) - the semen doesn’t contain any sperm. This may be caused by a blockage of the tubes, or testicular failure.
- Low sperm count (oligospermia) - the ejaculate has insufficient numbers of sperm to bring about conception.
- Abnormal shape - a healthy sperm is shaped like a streamlined tadpole. Abnormally shaped sperm may have problems penetrating the surface of the woman’s egg.
- Poor motility - a healthy sperm has a lashing tail, which helps it to swim through the woman’s reproductive system. Sperm with poor motility may swim feebly, or not at all.
Functional problems that can cause or contribute to male infertility include:
- Impotence - the inability to get or maintain an erection sufficient for sexual intercourse.
- Problems with the testicles - caused by injury, infection or chemotherapy.
- Prostatectomy - side effects of the surgical removal of the prostate gland, including infertility, impotence and incontinence.
- Certain disorders - such as multiple sclerosis or diabetes can cause erection and ejaculation difficulties.
- Antibodies - the man’s immune system makes antibodies that hinder the activity of sperm, such as reducing the sperm’s ability to latch onto the partner’s egg.
Hormonal problems
The levels of male sex hormones are regulated by a series of glands and their hormones. The pituitary gland in the brain influences hormone production in the testicles, under the guidance of another brain structure - the hypothalamus. A relatively uncommon cause of male infertility is the failure to make enough of the hormone gonadotrophin.
Idiopathic infertility
For around one in 10 couples investigated for infertility, no cause is found. This is called ‘unexplained’ or ‘idiopathic’ infertility.
Diagnosis methods
Investigating suspected infertility requires a number of tests for both the man and his partner. Diagnosing male infertility may involve:
- Semen analysis - a sample of the man’s semen is investigated in the laboratory and checked for abnormalities and the presence of antibodies.
- Blood tests - to assess hormone levels.
- Testicular biopsy - a fine needle and microscope are used to check the network of tubes to see if there are any sperm in them.
- Ultrasound test - to take pictures of the reproductive organs, such as the prostate gland.
There are no treatments available that can improve the quality of a man’s sperm. However, techniques can increase the odds of conception using the existing sperm quality. Treatment depends on the cause, but may include:
- Hormone therapy - if low sperm count is due to insufficient levels of the hormone gonadotrophin.
- Artificial insemination - the semen is collected and concentrated, then delivered with instruments directly into the partner’s uterus.
- In vitro fertilisation - conception occurs in the laboratory and the fertilised egg is implanted in the prepared uterus.
- Around 40 per cent of fertility problems originate in the man.
- Male fertility problems include poor quality sperm, low sperm count or blockages in the tubes of the reproductive system.
- Treatment options for poor sperm quality include artificially inseminating the partner with a concentrated sample of the man’s semen.
Labels: Infertility
Tuesday, October 27, 2009
Introduction
Any case of female infertility requires a careful and systematic anamnesis, which includes several questions that are generally not asked in the interview of most patients seen in a gynecological practice. It is after this important step that the necessary clinical investigations for the work-up of each given case can be selected in an appropriate manner in order to establish the correct diagnosis as precisely as possible and in the shortest length of time.
The three main questions to be answered are:
- Is the patient ovulating ?
- Are the conditions for implantation adequate ?
- Is the morphology of the uterus and the tubes normal ?
The answers are provided by the following methods:
Clinical evidence of ovulation
- Basal body temperature.
- Observation of the cervical mucus.
- Exfoliative vaginal cytology.
- Transvaginal sonography (ovarian follicles).
- Pituitary and ovarian hormones assays.
- Laparoscopy and direct observation of the ovaries.
Clinical evidence of readiness for uterine implantation
- Basal body temperature.
- Transvaginal sonography (thickness of the endometrium).
- Plasma progesterone assay.
- Endometrial biopsy.
- Hysteroscopy.
Clinical evidence of normality of the internal genital tract
- Hysterosalpingography.
- Transvaginal sonography.
- Hysteroscopy.
- Laparoscopy.
Clinical evidence of ovulation
Basal body temperature (BBT)
The early morning rectal temperature will rise approximately 0.5 to 0.7°C after ovulation and stay in a " plateau " for 12 to 14 days. This rise in BBT is due to a central effect of progesterone secretion. A slight drop of BBT might be observed 24 to 48 hours before ovulation, related to the estrogen peak secreted by the mature follicle.
Observation of the cervical mucus
Under the influence of the highest level of estrogen secretion from the dominant ovarian follicle, which precedes the ovulation, one can observe an abundant, clear and fluid secretion of mucus from the cervical canal. This transient secretion slightly but obviously dilates the external cervical os. It precedes ovulation by 4 to 2 days and is greatest on the day before ovulation. This mucus is highly receptive for the sperm penetration during sexual intercourse.
The cervical mucus disappears promptly after ovulation under the influence of progesterone secretion.
Exfoliative vaginal cytology
A vaginal smear, scraped from a lateral vaginal wall with an Ayres spatula or a wet cotton swab, provides a typical result at the time of ovulation, when examined under light microscope observation, after it has been stained with Papanicolaou or Schorr staining, or with any quick dye.
The superficial cells of the vaginal mucosa are flat, well scattered, with pyknotic nuclei and highly eosinophilic. As soon as ovulation has taken place, the cells become coiled, packed together and mostly basophilic .
Transvaginal sonography
The sonographic picture of a preovulatory follicle is well documented and typical. The mature follicle measures from 18 to 23 mm in average inner dimension.

After ovulation, the follicular wall becomes irregular and the fresh corpus luteum usually appears as a hypoechogenic structure and may contain some echoes corresponding to internal bleeding. The wall of the corpus luteum becomes thickened as the luteinization progresses .
Pituitary and ovarian hormone assays
The secretion of LH can be detected daily in urine samples by radioimmunoassay. The LH peak usually precedes ovulation by 48 to 24 hours. At the same time, the secretion of estrogen produced by the dominant follicle, reaches a maximum in the peripheral venous blood. Soon after ovulation, the level of progesterone in the peripheral blood rises from 2.5 to 4.0 ng/ml and reaches its maximum from day 5 to day 10 after the LH peak, with a variation from 7 to 12 ng/ml. This intermediate luteal phase is the physiological time for uterine nidation .
A schematic representation of the hormonal secretory patterns throughout the menstrual cycle is illustrated below
Laparoscopy
A mature follicle increases ovarian size considerably and looks like a round bluish cyst with one or two capillaries seen on its surface.
After ovulation, the stigma of the follicular rupture can be easily recognized as a small hole surrounded by an hemorrhagic structure on the surface of the ovary. Scars of previous ovulations can also be recognized on the surface of both ovaries. Clear yellowish follicular fluid can be found in the pouch of Douglas.
Clinical evidence of readiness for uterine implantation
Basal body temperature
A sustained " plateau " of 12 to 14 days following ovulation, is indicative of a good progesterone secretion from the corpus luteum, at least of 4 ng/ml in the peripheral blood.
Transvaginal sonography
The thickness of the secretory endometrium can be precisely measured. At its thickest, it reaches 8 to 14 mm, including both layers, and should be echogenic in a regular manner.
Plasma progesterone assays
In order to have a good evaluation of the secretion of the corpus luteum, one should obtain at least three to four blood samples, for instance every other day, starting from the third postovulatory day.
Endometrial biopsy
The tissue sample should be aspired either with a Novak cannula or with a plastic Cornier’s Pipelle around the time when nidation normally takes place, which means between day 20 to 22 of the cycle. Dating of the endometrial biopsy requires strict histological criteria (6).
Hysteroscopy
Using a small hysteroscope of 5 mm or 3 mm of diameter, an hysteroscopic examination of the uterine cavity can be easily performed on an out-patient basis in a clinic or in the office, with or without anesthesia. The examination can rule out the presence of uterine polyps, synechiae, or endometritis, all of which could interfere with nidation (5,15).
Clinical evidence of normality of the internal genital tract
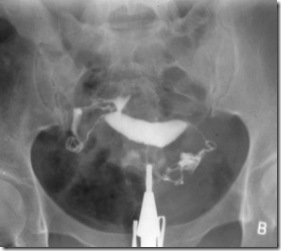
Hysterosalpingography
As in the case of other medical methods of investigation, strict technique is necessary in order to obtain precise information. A perfectly frontal view and also a good lateral view of the uterus, with a position of the uterus body being strictly parallel to the radiological film, is necessary to appreciate the size, the morphology and the outline of the uterine cavity.
A lateral view of a correct exposure of both tubes gives more information on their morphology than the frontal view. Also, the lateral view gives a better picture of the isthmic segment of the uterus and of its width in case of a suspected incompetence of the internal cervical os.
Until fibroscopic tools have been utilized enough and a sufficient optical knowledge on the inside morphology of the fallopian tubes has been accumulated, hysterosalpingography remains the only way to investigate the intramural segment and the isthmic segment of the fallopian tubes.
Pelvic adhesions can only be demonstrated by this radiological method, if a sufficient amount of opaque medium has been spread into the pelvis or, better, if a complementary hydrotubation with sterile saline is used at the end of the procedure, and if the last picture is taken after the patient has been leaned alternately on each side for a few minutes (" brassage ") .
Transvaginal sonography
With the use of a vaginal sound, we can now easily measure the size of the uterus, and observe the structure of the endometrium and of the myometrium. Polyps, myomas, internal synechiae and congenital malformations are well documented in specialized text books. Ovarian cysts and sactosalpinx can also be easily recognized with transvaginal sonography.
Hysteroscopy
With this method, using either CO2 gas or saline solution as a dilatation medium, the entire uterine cavity can be explored, and pathological findings detected, even those which can be sometimes missed with the hysterosalpingography.
The openings of the fallopian tubes in the uterine cavity can also be observed and demonstrated to be free of any obstacle as polyp or fibrotic tissue .
Laparoscopy
Trans- or paraumbilical laparoscopy remains the most complete method to explore the anatomical situation of both fallopian tubes and their relation with the adjacent ovaries. By means of direct optical observation, one can detect unsuspected peritubal and periovarian adhesions, or asymptomatic endometriosis, or agglutination of the fimbriae of the distal portion of the tubes.
With the advent of fine fibrotic catheters, introduced into the open fallopian tubes under laparoscopic control, we should be able to examine the internal appearance of the ampullary segments and detect small internal adhesions or post-inflammatory atrophy of the tubal epithelia .
References
- Bianchi, P.G., Rivest, R., Bischof, P., and Campana A. (1992): Bioactive-LH in the assessment of normal cycle phases. Abstracts from the 8th meeting of the European Society of Human Reproduction and Embriology. Hum. Reprod., A. 40, p. 28.
- Fleischer, A.C., and Kepple, S.M. (1992): Transvaginal Sonography. A Clinical Atlas. Pippincott Co., Philadelphia.
- Givens, J.R. (1978): Endocrine Causes of Menstrual Disorders. Year Book Medical Publishers Inc., Chicago.
- Gomel, V., Taylor, P.J., Yuzpe, A.A., and Rioux, J.E.: Laparoscopy and Hysteroscopy in Gynecologic Practice. Year Book Medical Publishers Inc., Chicago.
- Koss, L.G. (1992): Diagnostic and its Histopathologic Bases, 4th ed. J.P. Lippincott Co., Philadelphia.
- Rozin, S. (1965): Uterosalpingography in Gynecology. Thomas, Springfield.
- Siegler, A.M. (1967): Hysterosalpingography. Harper & Row, New York.
- Speroff, L., Glass, R.H., and Kase, N.G. (1983): Clinical Gynecologic Endocrinology and Infertility, 3rd ed. Williams and Wilkins, Baltimore.
- Tristant, H., and Benmussa, M. (1981): Atlas d’hystérosalpingographie, 2nd ed. Masson, Paris.
- Van der Pas, H., Van Herendael, B., Van Lith, D., and Keith, L. (1983): Hysteroscopy. MTP Press Limited, Boston.
- Wied, G.L., and Bibbo M. (1975): In: Gynecologic Endocrinology, edited by J.J. Gold. P.B. Hoeber, New York.
Labels: Infertility
Visitor
Labels
- Carcinoma (2)
- Dysmenorrhoea (4)
- Endometriosis (4)
- Female Genital Infection (3)
- Fluor Albus (4)
- General (3)
- Genital Prolapse (4)
- Gynecology (8)
- Infectious Disease in Pregnancy (13)
- Infertility (10)
- Menopause (3)
- Menstrual Disorder (10)
- Myoma Uteri (3)
- Obstetrics (13)
- Pelvic Floor (4)
- Pelvic Inflamatory Disease (3)
- Physiology of Human Reproductive System (4)
- Pregnancy (2)
- Sexually Transmitted Disease (6)
- Uterine Bleeding (4)
Followers
Facebook Badge
Copyright © 2009 Complicated Girl. All Rights Reserved.