Information Site About Reproductive System
About Me
Blog Archive
-
▼
2009
(58)
-
▼
October
(21)
- IN VITRO FERTILIZATION - IVF
- OVULATION PAIN - MITTELSCHMERZ
- SPERM ANALYSIS
- INFERTILITY TREATMENT - MALE
- INFERTILITY - MALE
- DIAGNOSTIC METHODS IN FEMALE INFERTILITY
- POLYCYSTIC OVARIAN SYNDROME
- INFERTILITY -FEMALE
- PUBERTY
- HOW TO GET PREGNANT
- MIGRAIN and HORMONAL CHANGES
- IRREGULAR VAGINAL BLEEDING
- TOXIC SHOCK SYNDROME
- THERAPEUTIC OPTION PREMENSTRUAL SYNDROME
- PREMENSTRUAL SYNDROME
- OVULATION
- MENORRHAGIA
- DYSMENORRHOEA
- AMENORRHOEA
- MENSTRUAL CYCLE
- REPRODUCTIVE ORGAN SYSTEM
-
▼
October
(21)
Tuesday, October 27, 2009
Introduction
Any case of female infertility requires a careful and systematic anamnesis, which includes several questions that are generally not asked in the interview of most patients seen in a gynecological practice. It is after this important step that the necessary clinical investigations for the work-up of each given case can be selected in an appropriate manner in order to establish the correct diagnosis as precisely as possible and in the shortest length of time.
The three main questions to be answered are:
- Is the patient ovulating ?
- Are the conditions for implantation adequate ?
- Is the morphology of the uterus and the tubes normal ?
The answers are provided by the following methods:
Clinical evidence of ovulation
- Basal body temperature.
- Observation of the cervical mucus.
- Exfoliative vaginal cytology.
- Transvaginal sonography (ovarian follicles).
- Pituitary and ovarian hormones assays.
- Laparoscopy and direct observation of the ovaries.
Clinical evidence of readiness for uterine implantation
- Basal body temperature.
- Transvaginal sonography (thickness of the endometrium).
- Plasma progesterone assay.
- Endometrial biopsy.
- Hysteroscopy.
Clinical evidence of normality of the internal genital tract
- Hysterosalpingography.
- Transvaginal sonography.
- Hysteroscopy.
- Laparoscopy.
Clinical evidence of ovulation
Basal body temperature (BBT)
The early morning rectal temperature will rise approximately 0.5 to 0.7°C after ovulation and stay in a " plateau " for 12 to 14 days. This rise in BBT is due to a central effect of progesterone secretion. A slight drop of BBT might be observed 24 to 48 hours before ovulation, related to the estrogen peak secreted by the mature follicle.
Observation of the cervical mucus
Under the influence of the highest level of estrogen secretion from the dominant ovarian follicle, which precedes the ovulation, one can observe an abundant, clear and fluid secretion of mucus from the cervical canal. This transient secretion slightly but obviously dilates the external cervical os. It precedes ovulation by 4 to 2 days and is greatest on the day before ovulation. This mucus is highly receptive for the sperm penetration during sexual intercourse.
The cervical mucus disappears promptly after ovulation under the influence of progesterone secretion.
Exfoliative vaginal cytology
A vaginal smear, scraped from a lateral vaginal wall with an Ayres spatula or a wet cotton swab, provides a typical result at the time of ovulation, when examined under light microscope observation, after it has been stained with Papanicolaou or Schorr staining, or with any quick dye.
The superficial cells of the vaginal mucosa are flat, well scattered, with pyknotic nuclei and highly eosinophilic. As soon as ovulation has taken place, the cells become coiled, packed together and mostly basophilic .
Transvaginal sonography
The sonographic picture of a preovulatory follicle is well documented and typical. The mature follicle measures from 18 to 23 mm in average inner dimension.

After ovulation, the follicular wall becomes irregular and the fresh corpus luteum usually appears as a hypoechogenic structure and may contain some echoes corresponding to internal bleeding. The wall of the corpus luteum becomes thickened as the luteinization progresses .
Pituitary and ovarian hormone assays
The secretion of LH can be detected daily in urine samples by radioimmunoassay. The LH peak usually precedes ovulation by 48 to 24 hours. At the same time, the secretion of estrogen produced by the dominant follicle, reaches a maximum in the peripheral venous blood. Soon after ovulation, the level of progesterone in the peripheral blood rises from 2.5 to 4.0 ng/ml and reaches its maximum from day 5 to day 10 after the LH peak, with a variation from 7 to 12 ng/ml. This intermediate luteal phase is the physiological time for uterine nidation .
A schematic representation of the hormonal secretory patterns throughout the menstrual cycle is illustrated below
Laparoscopy
A mature follicle increases ovarian size considerably and looks like a round bluish cyst with one or two capillaries seen on its surface.
After ovulation, the stigma of the follicular rupture can be easily recognized as a small hole surrounded by an hemorrhagic structure on the surface of the ovary. Scars of previous ovulations can also be recognized on the surface of both ovaries. Clear yellowish follicular fluid can be found in the pouch of Douglas.
Clinical evidence of readiness for uterine implantation
Basal body temperature
A sustained " plateau " of 12 to 14 days following ovulation, is indicative of a good progesterone secretion from the corpus luteum, at least of 4 ng/ml in the peripheral blood.
Transvaginal sonography
The thickness of the secretory endometrium can be precisely measured. At its thickest, it reaches 8 to 14 mm, including both layers, and should be echogenic in a regular manner.
Plasma progesterone assays
In order to have a good evaluation of the secretion of the corpus luteum, one should obtain at least three to four blood samples, for instance every other day, starting from the third postovulatory day.
Endometrial biopsy
The tissue sample should be aspired either with a Novak cannula or with a plastic Cornier’s Pipelle around the time when nidation normally takes place, which means between day 20 to 22 of the cycle. Dating of the endometrial biopsy requires strict histological criteria (6).
Hysteroscopy
Using a small hysteroscope of 5 mm or 3 mm of diameter, an hysteroscopic examination of the uterine cavity can be easily performed on an out-patient basis in a clinic or in the office, with or without anesthesia. The examination can rule out the presence of uterine polyps, synechiae, or endometritis, all of which could interfere with nidation (5,15).
Clinical evidence of normality of the internal genital tract
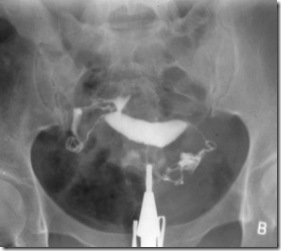
Hysterosalpingography
As in the case of other medical methods of investigation, strict technique is necessary in order to obtain precise information. A perfectly frontal view and also a good lateral view of the uterus, with a position of the uterus body being strictly parallel to the radiological film, is necessary to appreciate the size, the morphology and the outline of the uterine cavity.
A lateral view of a correct exposure of both tubes gives more information on their morphology than the frontal view. Also, the lateral view gives a better picture of the isthmic segment of the uterus and of its width in case of a suspected incompetence of the internal cervical os.
Until fibroscopic tools have been utilized enough and a sufficient optical knowledge on the inside morphology of the fallopian tubes has been accumulated, hysterosalpingography remains the only way to investigate the intramural segment and the isthmic segment of the fallopian tubes.
Pelvic adhesions can only be demonstrated by this radiological method, if a sufficient amount of opaque medium has been spread into the pelvis or, better, if a complementary hydrotubation with sterile saline is used at the end of the procedure, and if the last picture is taken after the patient has been leaned alternately on each side for a few minutes (" brassage ") .
Transvaginal sonography
With the use of a vaginal sound, we can now easily measure the size of the uterus, and observe the structure of the endometrium and of the myometrium. Polyps, myomas, internal synechiae and congenital malformations are well documented in specialized text books. Ovarian cysts and sactosalpinx can also be easily recognized with transvaginal sonography.
Hysteroscopy
With this method, using either CO2 gas or saline solution as a dilatation medium, the entire uterine cavity can be explored, and pathological findings detected, even those which can be sometimes missed with the hysterosalpingography.
The openings of the fallopian tubes in the uterine cavity can also be observed and demonstrated to be free of any obstacle as polyp or fibrotic tissue .
Laparoscopy
Trans- or paraumbilical laparoscopy remains the most complete method to explore the anatomical situation of both fallopian tubes and their relation with the adjacent ovaries. By means of direct optical observation, one can detect unsuspected peritubal and periovarian adhesions, or asymptomatic endometriosis, or agglutination of the fimbriae of the distal portion of the tubes.
With the advent of fine fibrotic catheters, introduced into the open fallopian tubes under laparoscopic control, we should be able to examine the internal appearance of the ampullary segments and detect small internal adhesions or post-inflammatory atrophy of the tubal epithelia .
References
- Bianchi, P.G., Rivest, R., Bischof, P., and Campana A. (1992): Bioactive-LH in the assessment of normal cycle phases. Abstracts from the 8th meeting of the European Society of Human Reproduction and Embriology. Hum. Reprod., A. 40, p. 28.
- Fleischer, A.C., and Kepple, S.M. (1992): Transvaginal Sonography. A Clinical Atlas. Pippincott Co., Philadelphia.
- Givens, J.R. (1978): Endocrine Causes of Menstrual Disorders. Year Book Medical Publishers Inc., Chicago.
- Gomel, V., Taylor, P.J., Yuzpe, A.A., and Rioux, J.E.: Laparoscopy and Hysteroscopy in Gynecologic Practice. Year Book Medical Publishers Inc., Chicago.
- Koss, L.G. (1992): Diagnostic and its Histopathologic Bases, 4th ed. J.P. Lippincott Co., Philadelphia.
- Rozin, S. (1965): Uterosalpingography in Gynecology. Thomas, Springfield.
- Siegler, A.M. (1967): Hysterosalpingography. Harper & Row, New York.
- Speroff, L., Glass, R.H., and Kase, N.G. (1983): Clinical Gynecologic Endocrinology and Infertility, 3rd ed. Williams and Wilkins, Baltimore.
- Tristant, H., and Benmussa, M. (1981): Atlas d’hystérosalpingographie, 2nd ed. Masson, Paris.
- Van der Pas, H., Van Herendael, B., Van Lith, D., and Keith, L. (1983): Hysteroscopy. MTP Press Limited, Boston.
- Wied, G.L., and Bibbo M. (1975): In: Gynecologic Endocrinology, edited by J.J. Gold. P.B. Hoeber, New York.
Labels: Infertility
Visitor
Labels
- Carcinoma (2)
- Dysmenorrhoea (4)
- Endometriosis (4)
- Female Genital Infection (3)
- Fluor Albus (4)
- General (3)
- Genital Prolapse (4)
- Gynecology (8)
- Infectious Disease in Pregnancy (13)
- Infertility (10)
- Menopause (3)
- Menstrual Disorder (10)
- Myoma Uteri (3)
- Obstetrics (13)
- Pelvic Floor (4)
- Pelvic Inflamatory Disease (3)
- Physiology of Human Reproductive System (4)
- Pregnancy (2)
- Sexually Transmitted Disease (6)
- Uterine Bleeding (4)
Followers
Facebook Badge
Copyright © 2009 Complicated Girl. All Rights Reserved.










0 comments:
Post a Comment